■ Definition of redox reaction
| ► | A process involves oxidation and reduction that occurs simultaneously. | |
| ► | In any redox reaction, there is only one oxidation process and one reduction process. |
| ► | loss or gain of hydrogen | |
| ► | loss or gain of oxygen | |
| ► | transfer of electrons | |
| ► | change in oxidation number |
| ► | A substance that is reduced in a reaction. |
| ► | A substance that is oxidised in a reaction. |
| ► | Oxidation: the process of losing hydrogen | |||
| ► | Reduction: the process of gaining hydrogen | |||
| ► | Example: In the redox reaction between hydrogen sulfide and chlorine gas.
|
| ► | Oxidation: the process of gaining oxygen | |||
| ► | Reduction: the process of losing oxygen | |||
| ► | Example: in the redox reaction between tin(IV) oxide (SnO2) and carbon (C)
|
Oxidation and reduction in terms of electron transfer
■ Oxidation and reduction in terms of electron transfer
| ► | Oxidation : the process of losing (releasing) electrons | |||
| ► | Reduction : the process of gaining (receiving) electrons | |||
| ► | The redox reaction can be defined as a process that involves electron transfer. | |||
| ► | The redox reaction can be defined as a process that involves electron transfer. | |||
| ► | Oxidising agent can be defined as an electron receiver. | |||
| ► | The following mnemonic method can be used to memorize the definition of oxidation and reduction in terms of electron transfer:
|
| ► | Redox reaction between sodium and chlorine gas.
|
|||
| ► | Redox reaction between magnesium and oxygen gas.
|
Oxidation and reduction in terms of change in oxidation number
■ Oxidation number
| ► | The charge that the atom of the element would have if complete transfer of electron take place. | |||
| ► | An atom of an element will have a positive oxidation number if it loses electron(s) easily. | |||
| ► | An atom of an element will have negative oxidation number if it obtains electron(s). | |||
| ► | The oxidation number is equal to the number of negative charge loses or obtains. | |||
| ► | Oxidation : the increase in oxidation number | |||
| ► | Reduction : the decrease in oxidation number | |||
| ► | Example: In the redox reaction between hydrogen sulfide and chlorine gas.
|
Oxidation number
■ Assigning oxidation number of elements in a compound.
| ► | The oxidation number of an atom in the elemental state is zero.
|
|||
| ► | The oxidation number of a monatomic ion is equal to its charge.
|
|||
| ► | The algebraic sum of the oxidation numbers in the formula of a compound is zero.
|
|||
| ► | The oxidation number of hydrogen in a compound is +1, except when hydrogen forms compounds called hydride with active metals, and then it is -1.
|
|||
| ► | The oxidation number of oxygen in a compound is -2, except in peroxides.
|
|||
| ► | The algebraic sum of the oxidation numbers in the formula for a polyatomic ion is equal to the charge on that ion.
|
| ► |
|
| ✍ Worked-example 3.1(a) Calculate the oxidation number of chlorine in each of the following ion.
|
| ✍ Worked-example 3.1(b) Calculate the oxidation number of manganese in each of the following compound/ion.
|
Naming compounds using the IUPAC nomenclature
■ Transition elements have more than one oxidation number.
| ► | Copper
|
|||||||||||||
| ► | Iron
|
|||||||||||||
| ► | Lead
|
| ► |
|
| ► |
|
Half reaction in redox reaction
■ In the redox reaction
| ► | Half of the reaction is an oxidising reaction and the other half of the reaction is a reducing reaction. | |
| ► | The equation for this half reaction is called a half equation (ion-electron equations). |
| ✍ Worked-example 3.1(c) A redox reaction occurs when magnesium, Mg, burns in oxygen gas, O2. 2Mg(s) + O2(g) → 2MgO(s) Solution: Each magnesium atom has lost two electrons to form magnesium ions, Mg2+. Half ionic equation: Mg → Mg2+ + 2e− (oxidation → loss of electrons) Each oxygen atom has received an electron to form oxygen ions O2−. Half ionic equation: O2 + 4e− → O2− (Reduction → electron received) |
Redox reaction
■ Redox reaction
| ► | Redox reaction is a reaction involving oxidation and reduction processes that occur simultaneously.
|
| ► | Write two half-equations. State the oxidising agent and reducing agent based on the two half-equations. | |
| ► | Write overall equation. | |
| ► | State the observation recorded. | |
| ► | State confirmatory test for the product of the reaction(optional). |
| ► | The reaction that do not involve change in the oxidation number. | |||||||
| ► | Example:
|
Redox reaction involving the combustion of metal in oxygen/halogen
■ Combustion of metal in oxygen
| ► | Metals like magnesium, zinc burn in oxygen to form metal oxides when heated strongly. | |
| ► | The combustion of metal and oxygen is a redox reaction. |
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Observation:
|
 |
Laboratory Activity 3.1.1 : The combustion of magnesium in oxygen |
Redox reaction involving the change of iron (II) to iron (III) ions
■ Iron(II) ions, Fe2+ and iron(III) ions, Fe3+
| ► | Ferum (iron) form two types of positive ions, which is iron (II) ions and iron (III) ions in the respective compounds. | |||||||
| ► | Colour of the iron (II) ions, Fe2+ solution is pale green, colour of iron (III) ions, Fe3+ is yellowish brown solution. | |||||||
| ► | Iron (II) ions and iron (III) ion in aqueous solution can be identified by chemical tests qualitative analysis.
|
| ► | Oxidising agents can be used in the changing of iron(II) ions, Fe2+ to iron(III) ions, Fe3+ . |
| ► | Acidified potassium dichromate (VI) |
|
| ► | Hydrogen peroxide |
|
| ► | Concentrated nitric acid |
|
| ► | Acidified potassium manganate (VII) |
|
| ► | Chlorine water |
 |
Laboratory Activity 3.1.2 : Change of iron (II) ions to iron (III) ions |
Redox reaction involving the change of iron (III) ions to iron (II) ions
■ Changing iron(III) ions, Fe3+ to iron(II) ions, Fe2+
| ► | Reducing agents can be used in the changing of iron(III) ions, Fe3+ to iron(II) ions, Fe2+ . |
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Zinc powder dissolves in the solution and the brown solution turn to green. | |||||
| ► | A green precipitate is produced when the sodium hydroxide solution is added. This confirm the presence of iron (II).
|
| ► | Hydrogen sulphide gas |
|
| ► | Stanum (II) chloride solution |
|
| ► | Sodium sulphite / potassium sulphite |
|
| ► | Sulphur dioxide gas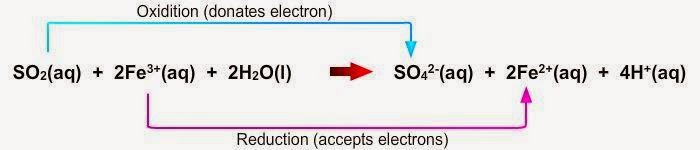 |
 |
Laboratory Activity 3.1.3 : Change of iron (III) ions to iron (II) ions |
Displacement reaction of metal from its salt solution as a redox reaction
■ Electrochemical series
| ► |  |
| ► | Higher metal in the electrochemical series have a higher tendency to form positive ions by losing electrons.
|
|||
| ► | Ions of metals which is located below the electrochemical series, the stronger is the oxidising ability of their ions. | |||
| ► | Metals which act as a strong reducing agents will have ions with weak oxidising agent, and vice versa. | |||
| ► | Thus, the metal at the top of the series is able to displace metals at the bottom of the series. |
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Observation:
|
|||||
| ► | Confirmatory test:
|
 |
Laboratory Activity 3.1.4 : Displacement of metals from its salt solution |
Displacement reaction of halogen from their halide solution by other halogen as a redox reaction
■ Halogen
| ► | Non-metallic elements in Group 17 of the Periodic Table. | |
| ► | Highly electronegative due to a high tendency to accept electrons. |
| ► |  |
|
| ► | Tendency to accept electrons into their halide ion decreases from top to bottom in Group 17. | |
| ► | A more reactive halogen (located at the top of the Group 17) can displace a less reactive halogen from its salts. |
| ► | The colour of halogens in their aqueous solution and the colour of aqueous solution with a little tetrachloromethane solution.
|
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Observation
|
Transfer of electrons at a distance as a redox reaction
■ Transfer of electron at a distance
| ► | When oxidising agent and reducing agent solutions is separated by an electrolyte in a U-Tube, redox reactions occur by transfer of electron using connecting wire. |
| ► |  |
|
| ► | Electrolyte acts as a salt bridge to separate two solutions but allows ions to pass through to complete the circuit | |
| ► | Electron transfer from reducing agent to oxidising agent through a connecting wire. | |
| ► | Carbon/graphite electrode that is immersed in reducing agent act as negative terminal. | |
| ► | Carbon/graphite electrode that is immersed in oxidising agent act as positive terminal. | |
| ► | The deflection of the galvanometer needle shows the electron flowing/moving. |
| ► | Solution that can react as oxidising agent:
|
|||||||||||||
| ► |
|
| ► |  |
|||||
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Observation:
|
| ► |  |
|||||
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Observation:
|
| ► |  |
|||||
| ► | Half ionic equation:
|
|||||
| ► | Overall equation:
|
|||||
| ► | Observation:
|
| ✍ Worked-example 3.1(a) State whether the following displacement reaction of metals from salt solution can occur.
|
| ✍ Worked-example 3.1(b) State whether the following displacement reaction of halogen from halide solution can occur.
|
| ✍ Worked-example 3.1(c) State whether the following chemical reaction are redox reactions.
|
| ⇲ For exercise(objective and subjective), download for free on Android OS. | |
 |
 |






Largest Source of India Potassium Sulphite Solution Manufacturers, Buy Variety of Potassium Sulphite Solution from Shakti Chemicals. Get Best Price and Quotations for Potassium Sulphite Solution. Contact Us: 9825043369
ReplyDelete